
Group number and symbol : They are determined by the type of element Periodic number : It is determined by the greatest principal quantum number ( n ) in the electronic configuration of the element.

Determination of the element location in the periodic table The periodic table shows a method to express the electronic configuration for the elements according to the nearest noble gas which precedes the element in the periodic table and this is considered the fourth method to the electronic configuration of elements.
Ħ4Gd : 1s², 2s², 2p 6, 3s ², 3p 6, 4s² , 3d 10, 4p 6, 5s², 4d 10 , 5p 6, 6s 2, 4f 7, 5d 1 The elements configuration in the light of the modern periodic table They are the elements of the f-block, They are characterized by having energy levels completely filled with electrons, except the three outermost levels. Ģ1Sc : 1s², 2s², 2p 6, 3s ², 3p 6, 4s², 3d 1 The inner transition elements They are the elements of the d-block, They are characterized by having energy levels completely filled by electrons, except the two outermost levels. ġs², 2s², 2p 6, 3s¹ → 1s², 2s², 2p 6 ( similar to the electronic configuration of neon gas 10Ne )ġs², 2s², 2p 6, 3s², 2p 4 → 1s², 2s², 2p 6, 3s ², 3p 6 ( similar to the electronic configuration of argon gas 18Ar )ġs¹ + 1s¹→ 1s² ( similar to the electronic configuration o f helium gas 2He ) The main transition elements They are active elements, because their highest level tends to reach the completed configuration similar to the nearest noble gas ( ns², np6 ) by gaining, losing or sharing electrons. They are the elements of s and p-blocks, except that of zero group, They occupy the groups from 1 A : 7 A, These elements are characterized by the complete filling of all the energy levels with electrons, except for the highest level. Noble gases may form compounds, but with great difficulty, because they are very stable elements as their energy levels are completely filled by electrons. They are the elements of the zero group 18 which is the last column of the p-block, They are characterized by having energy levels completely filled by electronsand their electronic structure is np 6, except that of helium 2He which is 1s². It is possible to classify the elements in periodic table into four types which are :
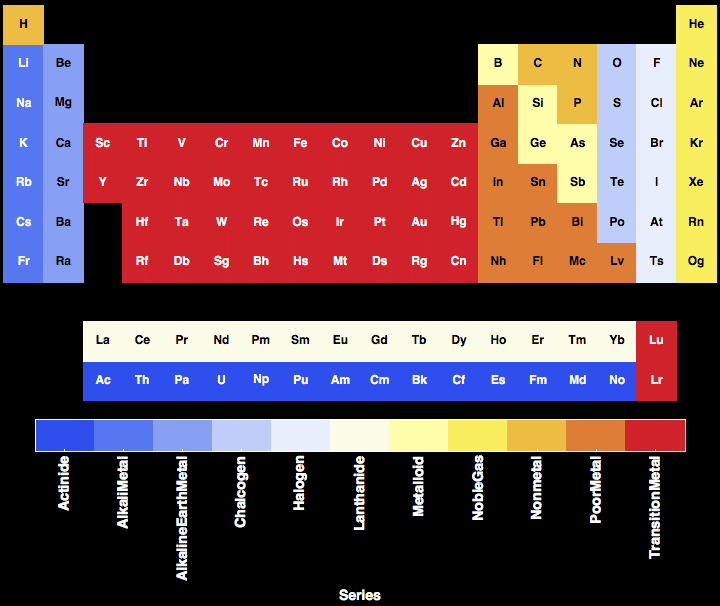
PERIODIC TABLE SERIES SERIES
The number of elements of any series of the f-block is 14 elements, while d-block contains 10 elements, because f-block elements in which the f-sublevel is successively filled and it consists of 7 orbitals, while d-block elements in which the d-sublevel consists of 5 orbitals and each orbital is occupied by 2 electrons. The actinides series : It is placed in seventh period, in which the 5f sub-level is filled successively, All the elementsof this series ar e radioactive and their nuclei are unstable.The lanthanides series : It is placed in sixth period, in which the 4f sub-level is filled successively, The elements of this series were named -wrong- by rare Earth’s elements, because they are quite similar in behavior and very difficult to be separated as the outermost energy level for all of the m 6s².They are separated down the table, to avoid being a very wide table, In which the f sub-level is filled successively, The f-block is divided into series – each of them includes 14 elements – which are : The third transition series : It includes the elements in which the 5d sub-level is filled successively, It lies in the sixth period and includes the elements from lanthanum ( 57La ) to mercury ( 80Hg ), excluding lanthanides.The second transition series : It includes the elements in which the 4d sub-level is filled successively, It lies in the fifth period and includes the elements from yttrium ( 39Y ) to cadmium ( 48Cd ).The first transition series : It includes the elements in which th e 3d sub-level is filled successively, It lies in the fourth period and includes the elements from scandium ( 21Sc ) to zinc ( 30Zn ).The d-block elements are classified according to the number of the outer energy level and the period number into three series which are : They occupy the middle block of the table, The d-block contains the elements with the outermost electronsoccupy the d sublevel, The d-block consists of 10 vertical columns representing 8 groups which are characterized by the symbol B except eighth group which consists of 3 vertical columns.


 0 kommentar(er)
0 kommentar(er)
